+
Global clients

Years Of
Experiences
At Tactus Lifesciences, our foundation is built upon the collective expertise and vision of seasoned pharmaceutical industry experts. Our operations have expanded to span over 27 countries across the globe, thanks to our trusted partners.
From our very inception, Tactus Lifesciences has been actively involved in sourcing Reference Listed Drugs (RLD), branded biologics, specialty medicines, and finished dosage formulations (FDF). With a unique blend of experience, strategic relationships, innovative approaches, and profound expertise in pharmaceutical regulatory affairs, we deliver unparalleled regulatory services to our clients with expert support throughout your product lifecycle from development to MAA renewal, globally.
We facilitate the in-licensing and out-licensing of pharmaceutical products to expand market reach and foster collaborations.
At Tactus Lifesciences, collaboration is at the heart of what we do. We are not just a service provider; we are your strategic partner in pharmaceutical success. Join us as we pave the way for innovation, collaboration, and excellence in the dynamic world of life sciences.
Enhance product commercialization globally with our Regulatory Strategy team, adept at ensuring regulatory compliance and market flexibility through agency collaborations.
Read MoreTactus Group delivers top-tier pharmaceuticals worldwide, specializing in branded, generic, specialty, controlled substance, and biosimilar products with extended shelf life.
Read MoreTactus Group facilitates seamless in- and out-licensing opportunities for pharmaceutical products, ensuring strategic partnerships and expansion of product portfolios.
Read More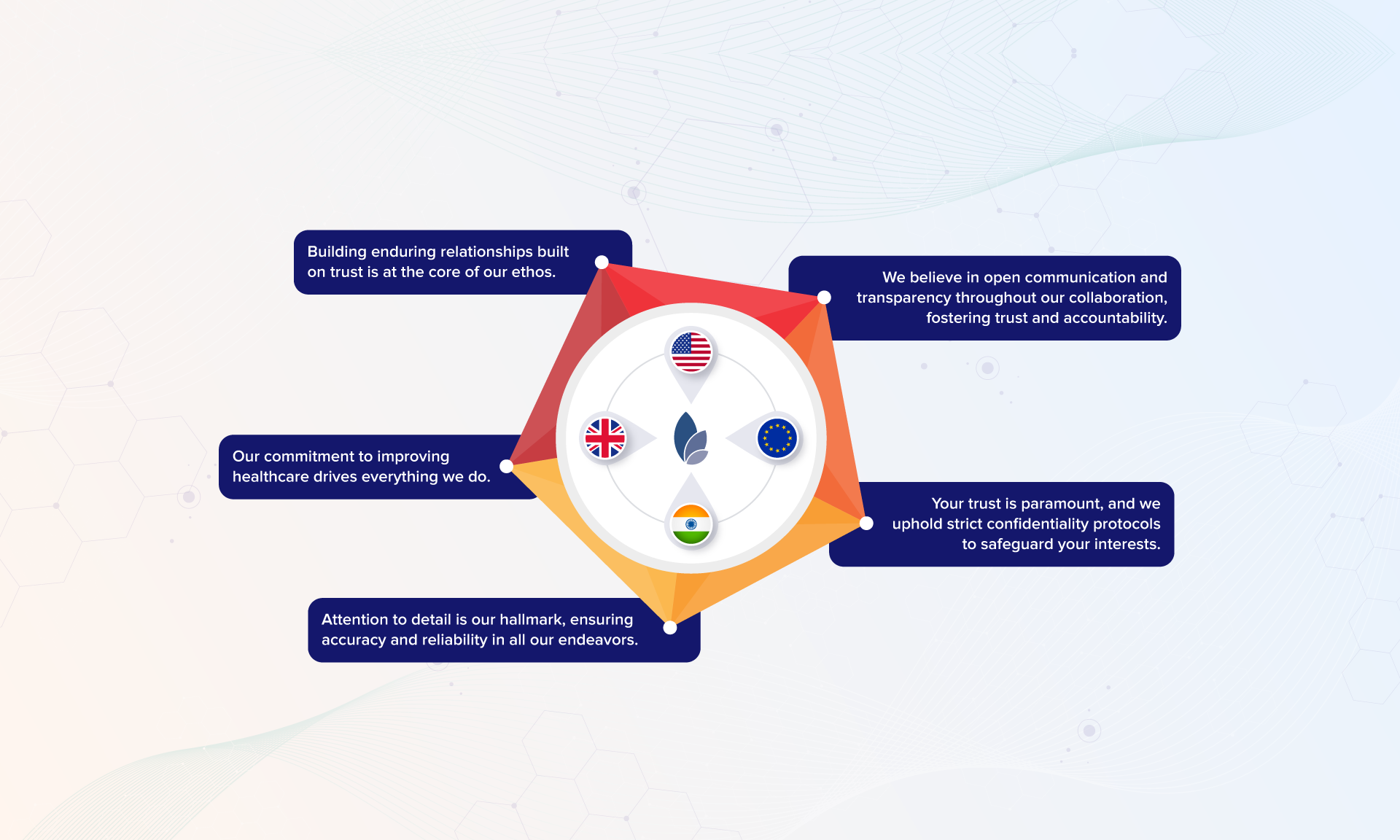
Global clients
Global presence