+
Global clients
Tactus Lifesciences has a strong global footprint, offering Regulatory Affairs (RA) and Pharmacovigilance (PV) services, along with GMP compliance and RLD sourcing, designed to meet diverse regulatory requirements across multiple markets. Our team of experts provides end-to-end support, from strategic dossier preparation and submissions to post-market surveillance, ensuring your products comply with the latest international regulations.
We guide clients through the regulatory frameworks of leading health authorities, including the FDA (USA), EMA (Europe), MHRA (UK), TGA (Australia), and Health Canada (Canada), helping to streamline approvals, accelerate market entry, and manage product lifecycles efficiently.
By partnering with Tactus Lifesciences, companies gain access to a trusted regulatory partner with deep expertise in global compliance, ensuring regulatory excellence, operational efficiency, and market readiness for pharmaceutical, biotech, and healthcare products.
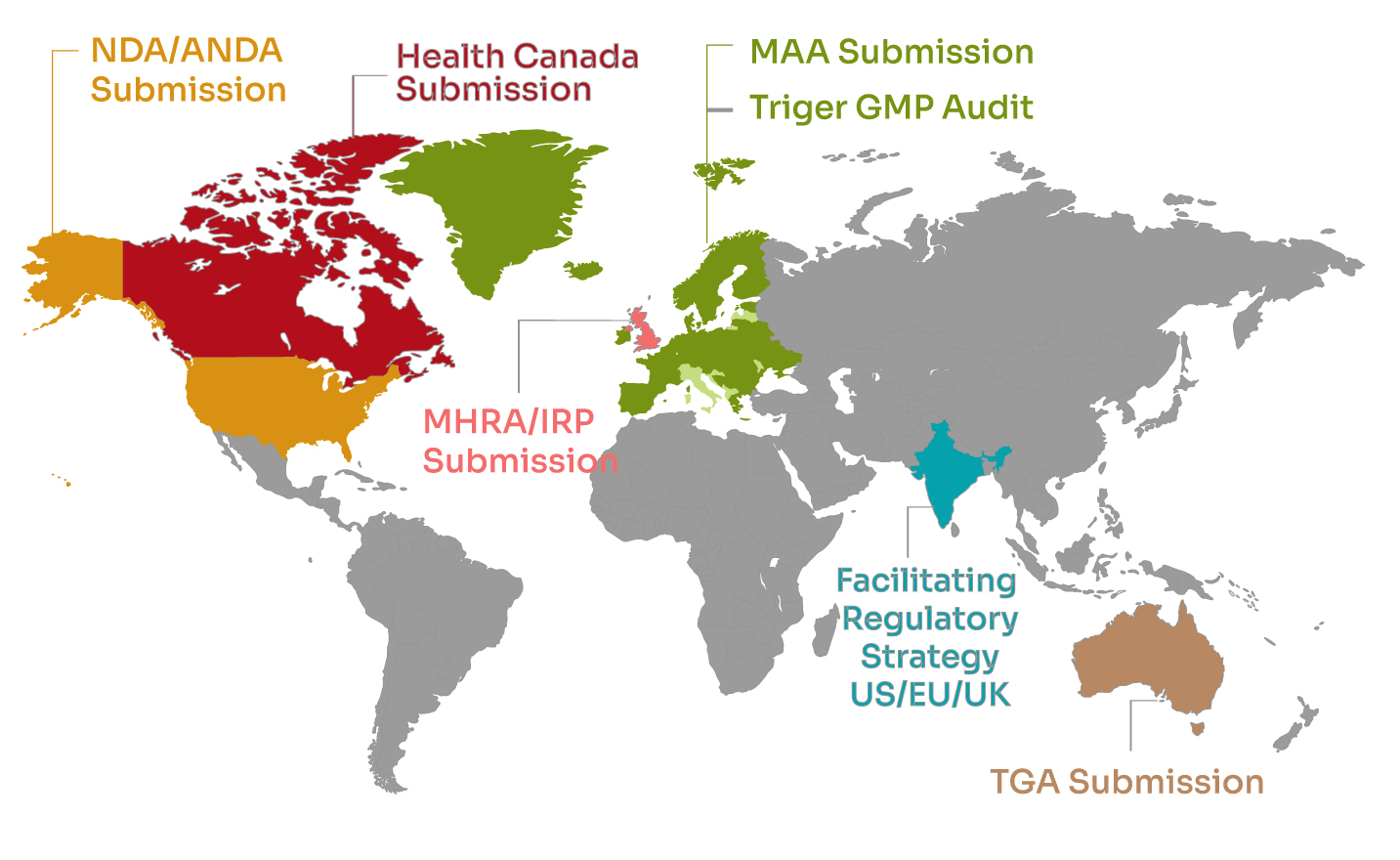
Global clients
Global presence