GMP Compliance And Audit Support
Empowering You with Quality-Centric GMP Solutions At Tactus, GMP compliance isn't just a checkbox—it's a strategic advantage.
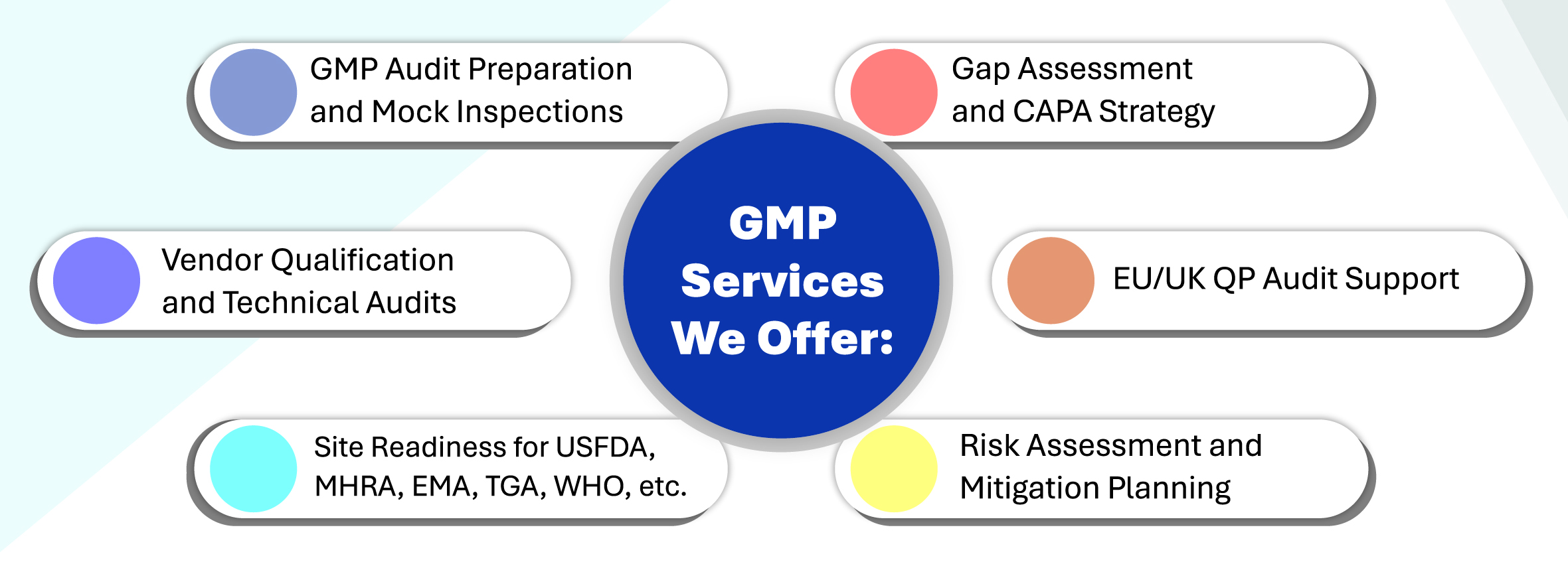
We support pharma companies with tailored GMP services for every stage of manufacturing and inspection readiness
Key Areas of Expertise:
- API & Finished Formulations (Sterile & Non-Sterile Facilities)
- Hormonal & High-Potency Facilities
- Clinical Trial Supply Chain (GCP/GMP Integration)
- GMP Documentation & Training Programs
Our GMP Edge
- QPs on panel
- 360° GMP health checks
- Concept-to-Commercial Pharma Solutions
- Region-specific guidance (EU Annex, US CFR, etc.)
- Global GMP compliance alignment for multi-site manufacturers

Integrating Quality with Speed
From product launches to facility expansions and partner due diligence, our GMP experts provide end-to-end support to ensure you remain audit-ready at all times. We combine deep regulatory expertise with a pragmatic approach, helping you achieve compliance without compromising on timelines.
Contact Us